Types of Neutron Interaction
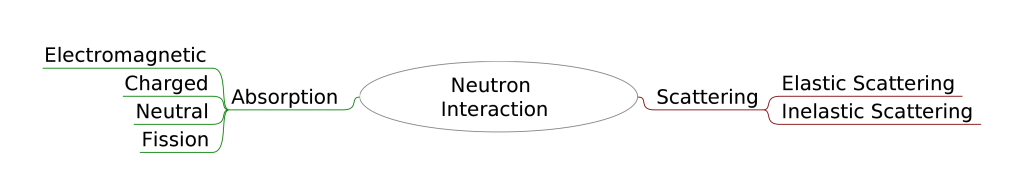
The neutron interaction is one among the major types Scattering and absorption.
In case of scattering, when the neutron interact with the nucleus the speed and direction of the neutron changes but the nucleus will be left with same number of neutron and proton as before. The energy imparted to the nucleus depends upon the angle of scattered neutron and mass of the nucleus. Eventually the nucleus will have some recoil velocity and will be in excited state that will result in the release of radiation.
The Scattering is subdivided into two types
- Elastic scattering
- Inelastic Scattering
Elastic Scattering
Elastic scattering is a dominant mechanism of energy deposition in case of High energy neutrons. During the interaction, as discussed earlier the fraction of neutron kinetic energy in transferred to the nucleus. The average energy loss for a neutron interacting with the nucleus of atomic weight A is from this expression its clearly understood that we need to use a material with low atomic number.
If we use hydrogen whose atomic weight A = 1 , the average energy lost will have highest value of E/2. i.e. if the neutron is 2 MeV , because of one single elastic collision it loses energy in average about 1 MeV. and in next collision in average it loses 0.5 MeV. It almost take 27 collision in this example before in becomes thermal neutrons i.e. 0.025 eV. As the atomic number is low as well as the mass of hydrogen is comparable to neutrons. The number of interactions to reduce the energy of neutron to desired level is less
Equation to find number of collision(n) in average required to reduce initial energy of neutron Eo to the desired energy En is
Inelastic Scattering
Inelastic scattering is same as that of elastic scattering expect here the nucleus undergoes internal rearrangement to go to excited state which eventually results in emission of radiation. The kinetic energy of the outgoing nucleus + neutron is less than that of incoming neutrons K.E. because some energy is lost in making the internal rearrangement of nucleus.
If the energy to make the nucleus go to excited state is very high it is highly unlikely that the inelastic collision takes place. It is the same case with hydrogen nucleus, they do not have excited states. so inelastic collisions are not possible. In general the scattering reduces(moderates) the energy of neutrons
Absorption
In case of low energy neutrons, the elastic scattering is not possible instead absorption or capture of neutrons takes place(, where the nucleus may rearrange its internal and emit one or more gamma rays or other charged particles such as protons, deuterons and alpha particles. the nucleus may also releases excess neutrons to become stable. The absorption of energy is greater than the energy of the emitted charged particles and neutrons.
Conclusion
These are the reasons why materials with low atomic numbers are used for neutron shielding which is contradictory to what we do for high energy photons, where we use high atomic number materials.
This is the reason why we use concrete barriers with combination of hydrogenous materials for construction of linac bunkers as they can shield photons, photo neutrons, and other charged particles and gamma rays that are produced while moderating neutrons. e.g. Neutron capture in hydrogen releases 2.224 MeV gamma rays.
We will look at the shielding calculations in future posts
If you want to ask any doubt or if you find any corrections to be made in this post please leave a comment below. thanks 🙂
Reference
Rinard, P. “Neutron interactions with matter.” Passive nondestructive assay of nuclear materials 375-377 (1991).