The process are divided into three phases
- Physical Phase
- Chemical Phase
- Biological Phase
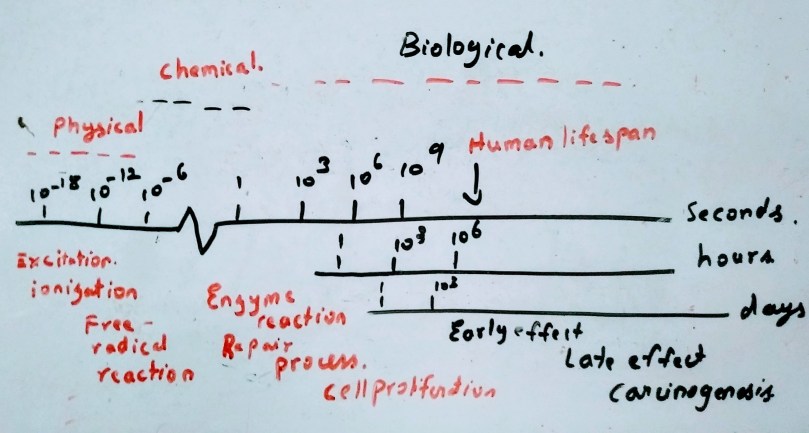
Physical Phase
In physical phase the interaction between the charged particle with the atoms of the tissues occurs. It mainly interacts with orbital electrons, ejecting some of them from atoms while exciting some electrons to higher energy levels. The secondary electrons with sufficient energy can induce further ionization and excitation along the tract(cascade of ionization events). In case of indirect action the fast electrons occurs in approximately 10-15 seconds. A high speed electrons takes about 10-18 seconds to traverse the DNA and 10-14 seconds to traverse the mammalian cells. for the volume of every 10 µm for 1 Gy of absorbed radiation dose almost 105 ionization events occur
Incident X-ray photon
⬇️
Fast electron 10-15 seconds
Chemical Phase
Fast electron
⬇️
Ion radical
⬇️
Free Radical
⬇️
chemical changes from breakage of bonds – 1ms(approx.)
About 80% of the cell in composed of water, when the radiation interacts with water molecules H2O H2O+ + e– . the H2O+ ion radical is formed. this ion radical further reacts with H2O to give H3O+ and OH* where OH* is a free radical. Free radical are highly reactive and they will undergo successive reaction to restore the electronic charge equilibrium. The free radical reactions are completed within 1 ms. The important process of chemical phase is the reactions which inactivate the free radicals e.g. reaction with sulphdryl compounds. Then the process of fixation reaction where the stable chemical changes are induced in biologically significant molecules. Here the fixation means not repair, it means that it made sure that the chemical change or damage is permanent.
Biological Phase
Chemical Changes from the breakage of bond
⬇️
Biological effect (days.months,years,
may not happen within human life span)
The biological effect occurs as the consequence of bonds broken. It begins with the enzymatic reaction that occurs on the residual chemical change. While vast majority of the cells repair, few may lead to cell death.
what is cell death? 1) loss of specific function - differentiated cells(nerve,muscles,secretory cells) 2) loss of ability to divide - proliferating cells such as stem cells 3) loss of reproductive integrity
During the first few week and months the loss of stem cells due to the radiation is the early manifestation of normal tissue damage and the early effects are also important for tumours as they are early responding tissues.
The secondary effect of cell killing is cell proliferation, which occurs both in tumours as well as normal tissues. for normal tissue it is an important mechanism as it reduces the acute side effect
The late reactions often occur after years of radiation exposure e.g. spinal cord damage, blood vessels damage and radiation carcinogenesis
what is ion radical?
ion means electrically charged and radical means having unpaired electron in valance shell.
H2O H2O+ + e–
H2O+ is a ion radical
What is free radical?
Free radicals do not have charge but have unpaired electron in the valance shell.
H2O+ + H2O H3O+ + OH*
OH* is the free radical
Reference
Joiner, Michael C., and Albert Van der Kogel. Basic clinical radiobiology fourth edition. CRC press, 2009.
[Most of the sentences written here are taken from basic clinical radio biology book. all credits go the authors of the book mentioned in reference ]